I bought a new coffee mug the other day. It will keep hot stuff, hot for 7 hours. It will keep cold stuff, cold for 18 hours.
Why the difference in times.
Heat moves to cold. I would think that the limited energy in the mug would transfer in less time, cooling the mug, than the practically infinite amount of energy available in the surrounding air, warming the mug, but apparently that's not the case.
Possibly the temperature differential? 32 to 70 vs 212 to 70
Help out the uneducated but curious.
Typically when you have cold liquids in the confined space you have added solid objects to maintain the coldness as long as possible. Hot liquids we as human tend to let chill to a drinkable temperature.....
Rate of change in temperature is proportional to the difference from ambient. The farther from ambient (positive or negative), the faster the temperature changes. So the time it takes for the temperature change to be readily perceived will be shorter for the more extreme temperature
Ranger50 hit it on the head. Most cold drinks will have ice in them. It takes more energy to melt ice, since it is going through a phase change, than to cool a liquid.
Toyman01 wrote: I bought a new coffee mug the other day. It will keep hot stuff, hot for 7 hours. It will keep cold stuff, cold for 18 hours. Why the difference in times. Heat moves to cold. I would think that the limited energy in the mug would transfer in less time, cooling the mug, than the practically infinite amount of energy available in the surrounding air, warming the mug, but apparently that's not the case. Possibly the temperature differential? 32* to 70* vs 212* to 70* Help out the uneducated but curious.
I am going to tl;dr the thread and blather on about my own interpretation.
It's easier to keep cold things cold than hot things hot because "hot" is generally 160-180F+ and "cold" is generally 60F -.
Room temperature is about 68F. Cold (unheated) water from the tap isn't much cooler than that, but it is generally acceptably "cold". So the difference between room temperature and "cold" is very minimal.
"Hot" on the other hand, as pertains to drinks, is typically around brewing temp. Which is in the 180F+ range. Don't believe me? Scald your hands on some 130F water straight from the hot tap, then pour yourself a mug of it, add some instant coffee or instant tea, and try to drink it. Blech, that's lukewarm!
In summary, this isn't a thermodynamics issue as much as it is a human perception issue.
More importantly, what mug? If it works even half as well as claimed, a great many of us would be interested in getting one.
In reply to foxtrapper:
http://www.gocontigo.com/mugs/20-oz-snapseal-superior-stainless-travel-mug.html

If you pour boiling water in it at 7am, it's still too hot to drink at 10am. It almost works too good.
It's the temperature difference and the fact that the difference from ambient for hot is greater than cold, like you already stated.
Consider this plot, but ignore the top, it's not relative to this discussion:
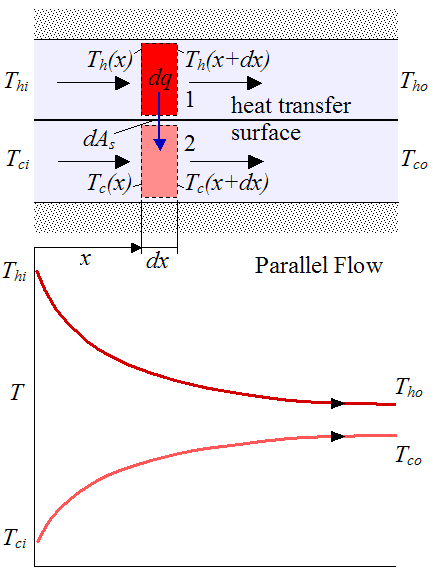
The top line would be the hot liquid and the bottom line would be the air right next to the mug. The farther the two temperatures the faster the heat moves. Say the hot liquid started at 212°F and no longer feels hot at 108°F and ambient is 70°F.
Conversely, for a cold liquid, it would be the bottom line and the top line would be the ambient at the surface of the mug. Now the cold liquid is at 32°F and ambient is 70°F again. The time it takes to get the ambient is the same amount of time it takes the 108°F to get to ambient but it's already 'barely' warm.
The temperatures would converge for either instance but since the plot is for a heat exchanger it doesn't show that but T_ho and T_co would be the same temp at some point.
I have a different Contigo travel mug and it does work almost too well. I will make coffee, put it in my mug and take it with me for my 45 min drive to the airport. It is hard to get the coffee to cool down enough to drink before the drive is over.
It's been a long time since I graduated engineering school. Now I just want to know if my drink is the correct temperature, not ponder why.![]()
Nice mug!![]()
I recently took a chemical engineering course for my second bachelors. I actually understood one of these threads. I'm going to pat myself on the back.
Thanks for the info on the cup. Significantly cheaper than that overpriced yeti nonsense. Just ordered a few for late christmas presents.
mith612 wrote: Rate of change in temperature is proportional to the difference from ambient. The farther from ambient (positive or negative), the faster the temperature changes. So the time it takes for the temperature change to be readily perceived will be shorter for the more extreme temperature
yup, this . So 34 F to 74 F is 40 degrees delta where coffee is usually 150 to 170 mark or ~110 to ~120 delta.
You'll need to log in to post.