
If you are just heating water in the drum, the speed at which the water moves will be of little consequence. Compare 2gpm vs 4gpm. The amount of heat offered to the water is fixed by the environment. Moving 2gpm might mean you can absorb 100 joules of heat into the water while it's in the exchanger. Moving 4gpm means you can only absorb 50 joules.... but you've moved double the water, so the temperature in the barrel will be the pretty much the same. This is why the old wive's tale of getting a high-flow water pump for your car almost never fixes an overheating problem. You are moving water faster which means it has less time to absorb heat from the engine, and a proportionally smaller amount of time to shed it in the radiator. It's mostly a wash.
If you're looking to plumb it through as an on-demand heater (that is, pumping the water from the barell, through the heat exchanger, and onto your body, there are a few zillion factors
Most of your heat will come from radiant sources (light). If it were dark outside and 70 degrees, the water would be 70 degrees. If it's the middle of a sunny 70-degree day, the water might be 100F.
So if you're planning on going barrel - solar heater - showerhead, it will take a lot of pretty intense calculations, and will vary widely if it's 50 and raining vs 100 and sunny. I think the value here would be to use the solar to heat the water in the barrel and then pull from the barrel to the showerhead.... so barrel - solar heater - barrel, and then barrel - shower.
One conundrum you might encounter depending on the size of the solar part. If you get a blistering hot streak (or as you in FL call it, "a warm spell"), the system you made to get warm water in the winter might end up boiling in the summer. I suppose you could add a pressure relief.
This is one of those builds that I think you're probably ahead of the game if you don't do the math, or at least if you do the math, don't expect accurate results. Even in the engineering world, you do all the calculations using the surface area of the tubing, the specific heat capacity of the copper/aluminum/plastic whatever, the real numbers you get in practice might vary widely.
A ten minute shower uses around 20 gallons on average, according to the interwebs. I think trying to heat up 55 gallons is going to be difficult, that's a lot of thermal mass.

SV reX
MegaDork
9/5/23 1:08 p.m.
A bucket bath can be done with about a half gallon of water... usually cold.
Not trying to be snarky. It's just what many people do.
Not sure if that works for you
You could use a thermosiphon instead of using a pump. Use the temp differential to move the water. Hot water in the collector will rise and flow into the tank. That will pull cold water from the tank into the collector to be heated. The temperature gradient will gradually move to the bottom of the tank. Pull your hot water off the top of the tank and add any cold water to the bottom.
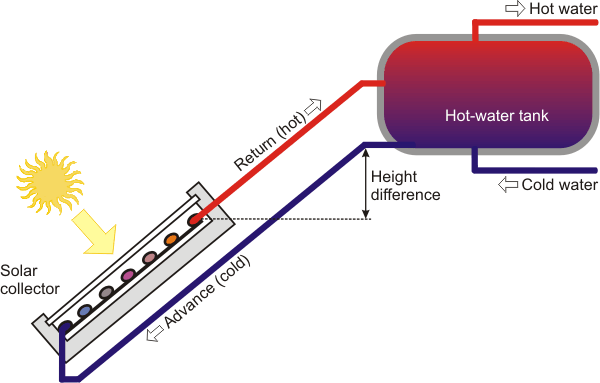
This may be worth a read as well.
https://www.greenpowerscience.com/WATER1.pdf
In reply to Toyman! :
That's interesting as I've thought about experimenting with thermosiphon to cool in the evenings. If the interior is warmer than outside letting the water raise to a higher level outside. Not sure how well it would work but I might do it on my shed to see.
codrus (Forum Supporter) said:
MrJoshua said:
Have you looked at using solar panels to directly power the elements in a hot water heater?
Energy is energy. If your goal is to heat water, you're not going to improve efficiency by converting the solar energy into electricity and then converting that back into resistive heat.
Google suggests solar water heater panels are about 80% efficient at turning solar energy into heat in the water. PV panels are barely a quarter of that.
This is some of the stupid stuff you find on YouTube. They have separate panels and a separate inverter just to run their hot water heater using PV. The only advantage I can see is it let's them run the hot water heater at lower voltage than normal. Seems like that money would have been better spent on more battery backup and just run the hot water heater off the main panels.
In reply to Stampie :
What you need to remember about Youtube videos is most of them are made just for the sake of being made. To stay at the top, they have to make content no matter how good it is.
When you do a search for solar there is a lot of chafe you have to sort through. It is the latest buzzword with all the negative connotations that go with it.
In reply to Toyman! :
Yeah I watched another video this morning where his automation and control systems probably account for a quarter of his electricity usage. Then I laughed at the end because he start talking about how he follows the KISS principle.

Mr_Asa
UltimaDork
9/7/23 12:05 p.m.
I keep meaning to sit down and actually play with this.
For some reason I'm just not interested in doing thermo homework for fun, even for Stampie.
In reply to Mr_Asa :
It's ok. I've given up on my original idea. When I build a full system I'll just overkill it with redneck engineering.

Mr_Asa
UltimaDork
9/7/23 12:10 p.m.
In reply to Stampie :
I was thinking of setting it up so you could play with area and such and figure out how much over-engineering you might need to do.
In reply to Mr_Asa :
Naw the redneck way is to build it bigger than you think you need then if that's not enough double down.

mtn
MegaDork
9/7/23 1:03 p.m.
100 foot hose, black, coiled with a window/plexiglass over it. Take advantage of the greenhouse effect. Run that to a 55 gallon drum, painted black. A pressure relief valve would probably be a good idea. Run another hose to the shower. Gut instinct is to say another 100 foot - kind of the Smokey Yunick line of thought - but that is probably ridiculous overkill.
This afternoon I remembered that I had a 15.5 gallon stainless potable water container sitting on my back porch. Time to experiment!
It was in the shade on the back porch so starting temp is 89 degrees.

Put it in direct sun for 30 minutes.

Ending temp is 113 degrees.

Now I know the whole thing wasn't 113 degress but I'll be back in a few with some redneck math.
A 15.5 gallon container like this weighs ~30 lbs.
15.5 gallons of water weighs ~125 lbs.
Since we know the whole container wasn't 113 degrees let's just half the rise in temp to be a delta of 12 degrees.
101 F x 30lbs = 3030 Flbs (told you it was redneck math)
89 F x 125lbs = 11,125 Flbs
(11125 Flbs + 3030 Flbs) / 155lbs = 91 F
A 2 degree rise in temp with 30 minutes of afternoon sun isn't bad considering it's not painted black, it's not in a sealed insulated box, and it's redneck math.
Fight me.
Stampie said:
A 15.5 gallon container like this weighs ~30 lbs.
15.5 gallons of water weighs ~125 lbs.
Since we know the whole container wasn't 113 degrees let's just half the rise in temp to be a delta of 12 degrees.
101 F x 30lbs = 3030 Flbs (told you it was redneck math)
89 F x 125lbs = 11,125 Flbs
(11125 Flbs + 3030 Flbs) / 155lbs = 91 F
A 2 degree rise in temp with 30 minutes of afternoon sun isn't bad considering it's not painted black, it's not in a sealed insulated box, and it's redneck math.
Fight me.
https://en.wikipedia.org/wiki/Specific_heat_capacity
Specific heat of aluminum is 0.9 J/g-K, whereas that of water is 4.184. So it takes about 4.5 times as much energy to heat 1 gram of water by 1 degree as it does 1 gram of aluminum.
In reply to codrus (Forum Supporter) :
Low blow. Good point.

mtn
MegaDork
9/7/23 5:45 p.m.
Stampie said:
It was in the shade on the back porch so starting temp is 89 degrees.
Sounds like the problem is solved, your water is heated.
In reply to mtn :
It's been cool this week.
codrus (Forum Supporter) said:
Stampie said:
A 15.5 gallon container like this weighs ~30 lbs.
15.5 gallons of water weighs ~125 lbs.
Since we know the whole container wasn't 113 degrees let's just half the rise in temp to be a delta of 12 degrees.
101 F x 30lbs = 3030 Flbs (told you it was redneck math)
89 F x 125lbs = 11,125 Flbs
(11125 Flbs + 3030 Flbs) / 155lbs = 91 F
A 2 degree rise in temp with 30 minutes of afternoon sun isn't bad considering it's not painted black, it's not in a sealed insulated box, and it's redneck math.
Fight me.
https://en.wikipedia.org/wiki/Specific_heat_capacity
Specific heat of aluminum is 0.9 J/g-K, whereas that of water is 4.184. So it takes about 4.5 times as much energy to heat 1 gram of water by 1 degree as it does 1 gram of aluminum.
But sir I failed to notice an error. This is stainless steel not aluminum. Don't know if that helps me or not cause I'm not smart enough.

Mr_Asa
UltimaDork
9/7/23 6:22 p.m.
In reply to Stampie :
Al, going by memory, has a higher specific heat than most other metals. So you now have even more of a gap
Mr_Asa said:
In reply to Stampie :
Al, going by memory, has a higher specific heat than most other metals. So you now have even more of a gap
Indeed. https://theengineeringmindset.com/specific-heat-capacity-of-materials/
316 stainless has a SHC of 0.5, so it's almost double the difference from aluminum. And yeah, that keg probably isn't 316, but the number is probably fairly close.
Water has one of the highest SHCs of any common material. The only two in that list that are higher are hydrogen and helium, and the only metal that's even close (half) is lithium.
In reply to codrus (Forum Supporter) :
So what I'm hearing is let's try it and see what happens.

Mr_Asa
UltimaDork
9/7/23 8:33 p.m.
With specific heat, you've got half of it.
Other half is that the sun outputs roughly 1500 watts per square meter.
In reply to Mr_Asa :
At the end of this I'll be asking the Sun who's it's daddy.






































